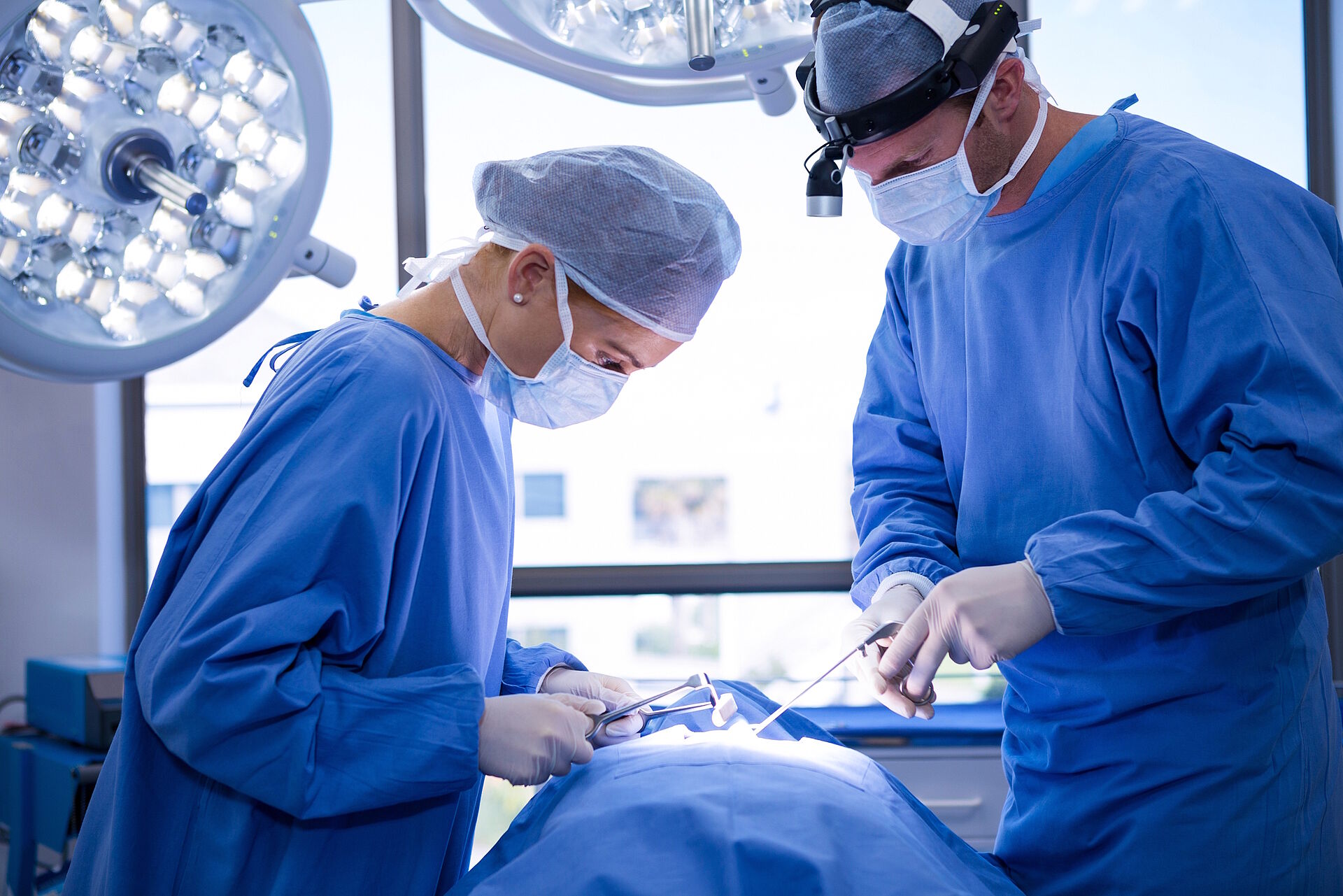
Particularly high demands are placed on surgical textiles. The transmission of infectious agents between hospital staff and patients during surgical and other invasive procedures should be minimised as far as possible. By fulfilling basic requirements, the textiles contribute to the general safety of patients.
Hohenstein tests your products for compliance with the performance requirements of single-use and reusable surgical drapes and gowns for use as medical devices according to the specifications of the European standard 13795-1:2019 or clean-air garments according to DIN EN 13795-2.
Biological tests
- Resistance to germ penetration in moist condition according to DIN EN ISO 22610
- Resistance to germ penetration in dry condition according to DIN EN ISO 22612
- Evaluation of cleanliness microbial/bioburden and of biocompatibility (EN ISO 11737-1)

Physical tests
- Particle release in dry condition (linting) according to DIN EN ISO 9073-10
- Resistance to liquid penetration according to EN ISO 811
- Bursting strength in in dry and wet state according to EN ISO 13938-2*
- Tensile strength in dry and wet state according to EN ISO29073-3
* Deviating from the requirement standard DIN EN 13795-1, the burst strength is not carried out according to EN ISO 13938-1, but according to EN ISO 13938-2. EN ISO 13938-1 section 1 indicates that there is no significant difference in the bursting strength results achieved using tests according to EN ISO 13938-1 (hydraulic method) and EN ISO 13938-2 (pneumatic method) for pressures up to 800 kPa.