When used correctly, your medical device must not pose any avoidable risks to patients, users or third parties.
You as the manufacturer or your authorised representative are responsible for this and confirm this in the EU Declaration of Conformity to the Medical Devices Regulation (EU) 2017/754. The basis for this is the technical documentation, which includes the General Safety and Performance Requirements (GSPR) and the risk assessment for your product. The technical documentation for your product therefore requires a considered approach - from planning to reporting.
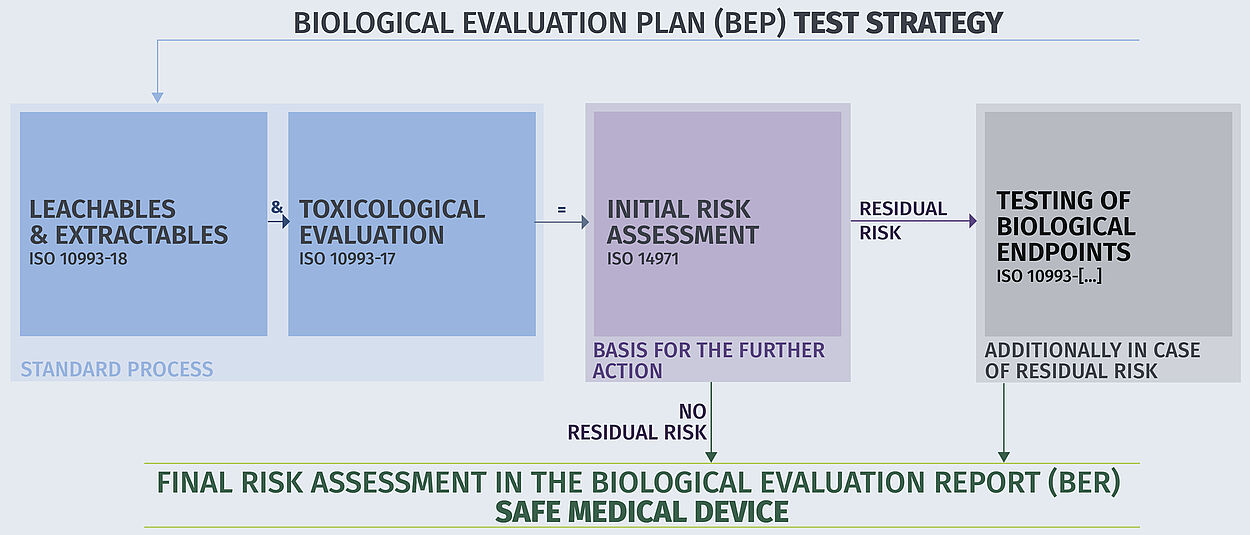
The basis for the biological assessment of medical devices is the DIN EN ISO 10993 series of standards. DIN EN ISO 10993-1 specifies which endpoints you need to consider in the biological risk assessment of your product. - You can also use our Product Finder!
How can our tests support you?
Our test results support the documentation and evaluation of important aspects of the biocompatibility of your medical device - from material selection, toxicological risk assessment (TRA) and material properties even after ageing or reprocessing (cleaning and sterilisation processes in accordance with ISO 17664) through to post-market surveillance of the product.